How can we improve healthcare innovation adoption in Catalonia? Digital health therapeutics companies respond
We get a first-hand look at the experience of Catalan digital therapeutics companies (DTx) with the healthcare system and take advantage to ask what needs to improve, in their opinion, to facilitate adoption of innovative projects like theirs.

In our last post on innovation adoption in the healthcare system in Catalonia we talked about the main challenges facing DTx companies in Europe, above all with issues like assessment and reimbursement. We also looked at Catalonia’s commitment to not be left behind and work in the same line as these countries to adopt these and other innovations in the Catalan healthcare system.
Carrying on in the same line, we spoke with a group of Catalan DTx firms to find out about their interactions with the system and their opinions about what should be done to improve the healthcare innovation adoption process and access to new medical technology in Catalonia.
Don’t miss their experiences and proposals we’ve compiled in this post!
Months of negotiations with each center
In most cases, the first thing a DTx company does when it wants to access the Catalan system is to speak with the healthcare centers. One by one. After many presentations, negotiations and long waits, some manage to tie down collaboration deals with some centers. One example is Nixi for Children, as company CEO Tomàs Lóbez explains, “We just closed a collaboration agreement with Vall d’Hebron University Hospital, which we started working on in 2019.”
Another company with a similar experience is the DyCare rehabilitation platform ReHub®, which has been adopted by the Vall d’Hebron University Hospital, Hospital de la Santa Creu i Sant Pau and Consorci Sanitari Integral. “The experience has been positive,” notes co-founder and CEO Silvia Raga, “but the time it took for them to acquire the tool could have been better: between six and nine months!”
Mireia Cigarran, founder of VRPharma, is currently waiting it out, hoping for months for a response so they can sign new collaboration agreements with new centers. “Our situation is that they want to adopt the technology, its value is very clear and the healthcare team values it, but they can’t implement it because it hasn’t been assigned a budget item for reimbursement,” the entrepreneur notes. “Digital startups need things to move quickly to take advantage of our momentum.”
Working magic to adopt innovation
Hospitals don’t have a budget earmarked for procuring innovation and are forced to juggle the existing one to implement these technologies. This is the case of Hospital Clinic, where they incorporate digital solutions in exchange for clinical validation or royalties on sales, explains Laura Sampietro, head of Innovation and Health Technology Assessment. This center also has an mHealth Observatory, which has analyzed over 35 mobile apps developed by hospital professionals to foster access to new digital solutions. Hospital Clinic is currently using a DTx from Baxter for patients who need hemodialysis.
At Hospital Universitari Parc Taulí, the type of economic collaboration depends on whether it is Parc Taulí that needs to implement a digital solution, and therefore needs a company to co-develop a new product, or if the company approaches the hospital to do clinical validation of their solution.
In the first case, so both parties can benefit from the collaboration, Parc Taulí and the chosen company work together to apply for competitive funding or innovative public procurement to get royalties after they develop the technology jointly. For clinical validation, the company has to pay fees to the center to cover the cost of resources, facilities and staff, or the hospital takes a stake in the company and future royalties.
“Collaborations can last between 1 and 3 years, depending on whether it is a clinical validation or technological development,” explain Eduard Soler, head of Innovation at the Parc Taulí Research and Innovation Institute, and Ismael Ávila, project manager at the same center. Parc Taulí has developed three DTx in mental health, including Brainhealth Solutions, created jointly with Institut Gutman, which has obtained CE marking and is on the market.
The US market, a faster, more affordable alternative
In the United States, the DTx industry is growing quickly, above all thanks to the FDA’s proactive encouragement of these therapies through its Digital Health Center of Excellence. And not just that, the American market also acts as a single market, with mutual recognition of certification and standardized clinical evidence. It has everything the European market doesn’t.
“After making the rounds of Catalan hospitals for four years and not getting any clear answers, we’ve decided to try our luck in other markets,” explains Braingaze CEO Laszlo Bax.
One company that has landed in the US recently is Amelia Virtual Care* , and it’s not going half bad, explains founder and CEO Xavier Palomer, “40% of our turnover is already from the United States.” The entrepreneur turned to this market and the private sector due to the fragmentation and lack of agility in adoption in Europe. “The European public health system is a ‘startup killer’,” he said at a recent debate.
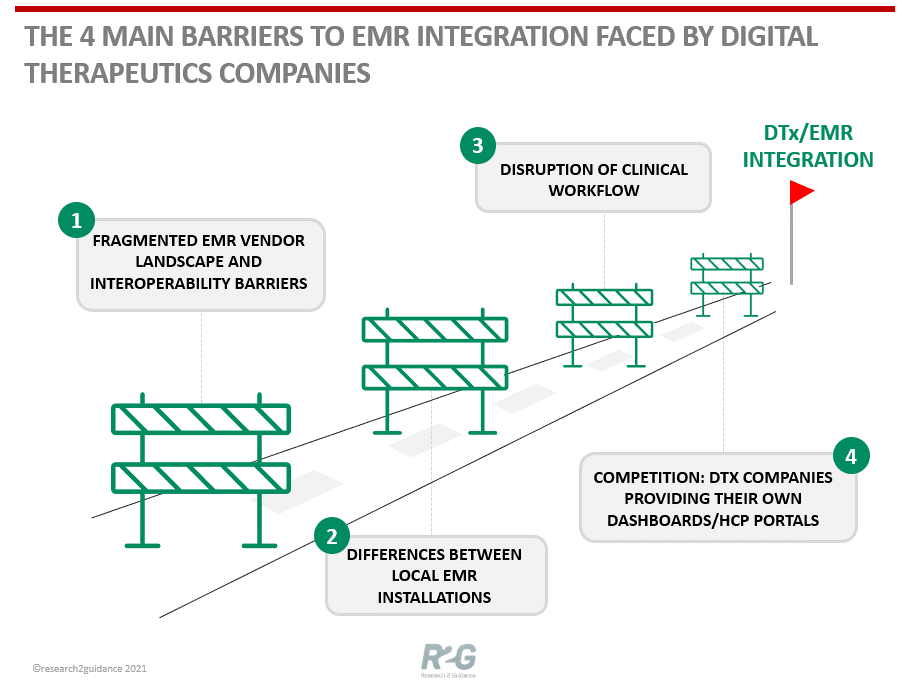
Source: Why are digital therapeutic companies not connecting to the traditional healthcare system by integrating with an EMR. Research 2 Guidance.
10 proposals to accelerate adoption of innovation in the Catalan system
To prevent these startups from being diluted before the technology can reach patients, Catalan DTx firms have some proposals we’ve compiled here:
1. Have one organization to manage access to the system
Silvia Raga of DyCare and Renalyse co-founder and CEO Adrià Maceira recommend centralizing DTx adoption in one organization, drafting joint action plans with the companies and clearly laying out the stages for entering the system. "You don't know who to call or who to approach to be able to present your project," adds Eva Garcia, co-founder and CEO of WIVI Vision.
2. Standardize clinical assessment
Some startups warn: “The lack of specific Europe-wide regulation for these therapies means they are still evaluated like any other medical device. And in Spain the situation is even more complex because assessment falls to the individual autonomous communities.”
3. Adapt compensation
“Each department decides how much it can or will pay for the solution, regardless of whether the price even covers production costs,” explains Erika Barrera of Virtual Bodyworks, a solution working with the Catalan administration to provide rehabilitation services at corrections centers run by the Department of Justice.
4. Establish a specific, flexible regulation
Silvia Raga of Dycare calls for “a regulation adapted to software, flexible and agile, like the product, which is always evolving.”
5. Earmark a specific budget for innovative public procurement
“A lot more could be done to strengthen innovative public procurement,” notes David Hurtado, head of Marketing at Brainhealth Solutions. He believes the excessive red tape and complex processes lead many centers to shoehorn DTx in any way they can.
6. Earmark a budget for commercial pilot programs to validate public use
María Jesús Salido, co-founder and CEO of Social Diabetes, believes an initial market is necessary for commercial pilot testing to validate public use and show the benefits of the products and solutions.
7. Encourage companies in the very early stages
“We have to break down the barriers that hamper startups compared to big companies, which tend to be the usual suppliers,” says Elisabet del Valle, CEO and co-founder of Onalabs.
8. Improve the skills of healthcare professionals in digital solutions
“Healthcare professionals need to understand the benefits of digital solutions and believe in the digitalization of healthcare,” says María Jesús Salido of Social Diabetes, explaining that specific training in this field could improve the situation.
9. Design prescription protocols for solutions not integrated into the system
Maria Barruezo, co-founder of LactApp, proposes prescription protocols be drawn up for digital solutions that don’t end up being integrated into the system. “This way, healthcare professionals can recommend them with confidence.”
10. Digitalize the healthcare system
Laszlo Bax of Braingaze proposes digitalizing the system to make it more agile. And he proposes using the Next Generation EU funds to do so.
Source: Compiled internally based on feedback from a group of Catalan DTx companies. Biocat
Nevertheless, many DTx firms in the BioRegion are happy to be part of an ecosystem with great capacity for innovation and talent development for entrepreneurs. “Public agents like ACCIÓ, Biocat, CIMTI, Barcelona Activa, Tic Salut and organizations like Barcelona Health Hub and Tech Barcelona, among others, bring key value for starting up, mentoring and accelerating new projects in healthcare with great social and economic impact,” notes Mireia Cigarran of VRPharma.