Catalonia is ranked 6th in number of trial participations in Europe
<p>Catalonia is participating in over 1,200 clinical trials currently underway to test new therapies and drugs in patients. </p>
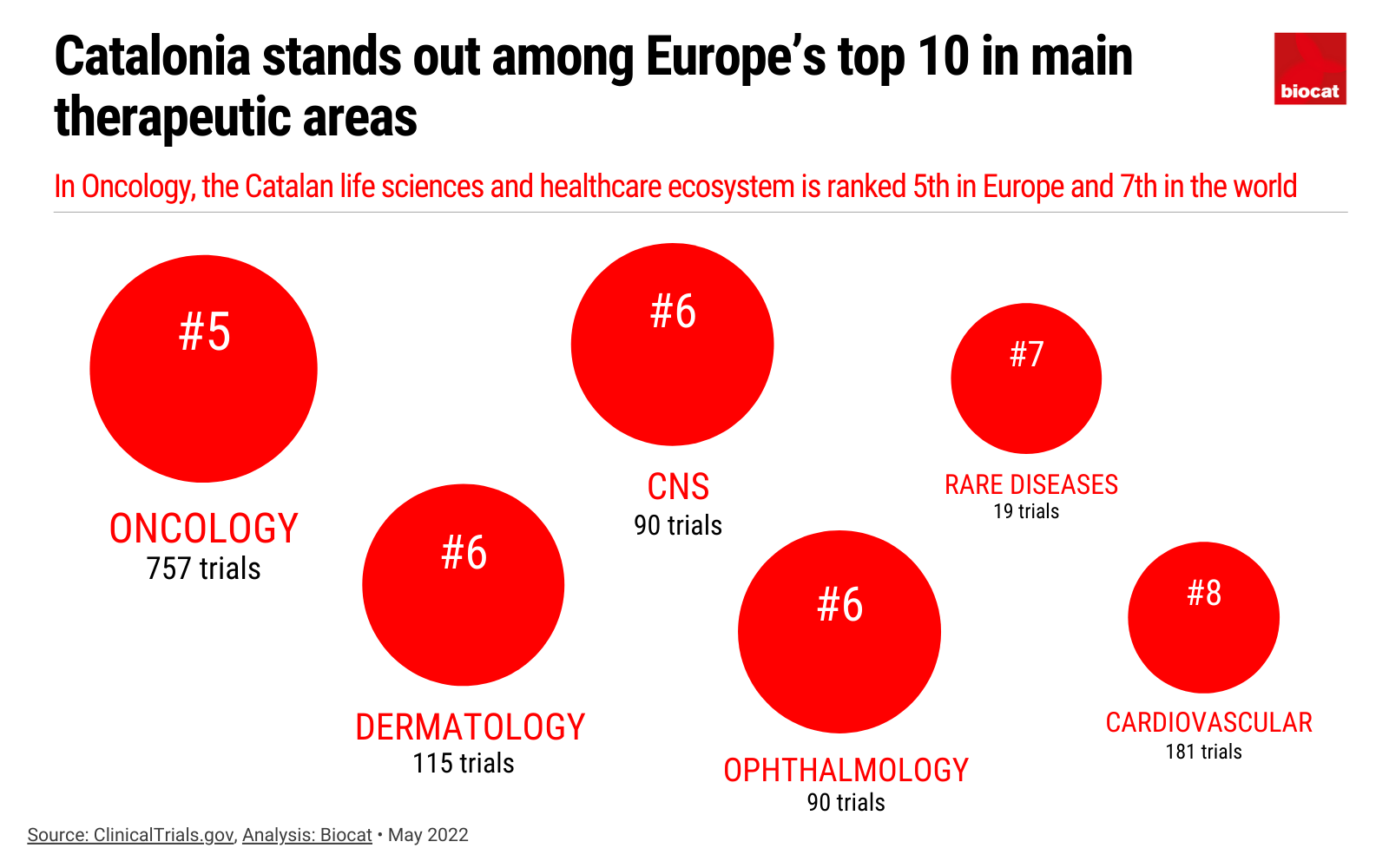
<p>The BioRegion stands out among Europe’s top 10 in main therapeutic areas.</p>
<p>Biocat releases these data coinciding with the International Clinical Trials Day, which is celebrated every year on May 20. </p>
Barcelona, 18 May 2022.- Catalonia is participating in over 1,200 clinical trials currently underway to test new therapies and drugs in patients. This puts the BioRegion 6th in Europe in number of trial participations and 8th in the world.
Catalonia is only surpassed in number of participations in active studies by large countries like the United States (9,532), Canada (1,905), France (1,896), United Kingdom (1,633), Spain (1,595), Germany (1,583) and Italy (1,460). The data also shows that Catalan centers take part in 75% of all active trials run in Spain, according to Clinical Trials data analyzed by Biocat (may 2022).
In terms of therapeutic areas, Catalonia excels in clinical trials in a wide range of specialities but particularly stands out in one: oncology, which is the focus of 65.5% of all clinical trials with Catalan participation, followed by cardiovascular diseases (15.7%), dermatology (13.4%), diseases of the central nervous system (7.8%) and ophthalmology (7.8%). In fact, Catalonia stands out among Europe’s top 10 in main therapeutic areas and, in oncology, the Catalan life sciences and healthcare ecosystem is ranked 5th in Europe and 7th in the world.
48% of the clinical trials in which Catalan centers participate are in phase I (15%) or phase II (33%), and 52% in phases III (49%) and IV (3%), i.e. the phases closest to drug commercialization. By centers, Vall d’Hebron University Hospital is the most active in clinical trials, followed by Hospital Clinic Barcelona, Bellvitge University Hospital, Hospital de la Santa Creu i Sant Pau, Hospital Universitari Parc Taulí and Parc de Salut Mar.
For Biocat CEO Robert Fabregat, “Catalonia has become one of the main driving forces for clinical trials in the world in recent years.” This can be seen in facts like, for example, that “since 2001, the number of clinical trials in Catalonia has grown every year, except 2020 when growth stagnated due to the COVID-19 pandemic.” Nevertheless, the CEO of Biocat believes, “The figures are very positive and maintain pre-pandemic levels.”
The main assets that explain why Catalonia has become a priority destination for clinical trials worldwide are: Top-notch healthcare system with access to a large population and an excellent primary care network, first-class hospitals, costs are more competitive than in other European countries and the United States and presence of world-renowned Key Opinion Leaders (KOLs) in various therapeutic areas, such as doctors Josep Tabernero in oncology, Jordi Bruix and Josep Maria Llovet in hepatology, Antoni Bayes-Genis in Cardiology, Bonaventura Clotet in HIV, Merce Boada in Alzheimer's and Xavier Montalban in multiple Sclerosis.
One example of this attraction is the clinical trials run in Catalonia by the top international pharmaceutical companies with the highest turnover, like Novartis, Roche, MSD, AstraZeneca, Janssen, GSK, Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, Gilead, Pfizer, AbbVie and Sanofi; and the recent announcement from Alexion, owned by AstraZeneca, which will open a new center for clinical trials in rare diseases in Barcelona is 2022.
This is one of the reasons an important international event like the Summit for Clinical Trials Operations Executives Europe (SCOPE Europe) chose the Catalan capital for their 4th annual congresses, held last month, and that Barcelona has just been named host of the upcoming international conference on biotechnology BIOSPAIN 2023.
Biocat releases these data coinciding with the International Clinical Trials Day, which is celebrated every year on May 20 to commemorate the day James Lind started the first study of this type, in 1747. This date is a reminder of the importance of health research in finding new ways to improve treatments and the quality of life of people with disease.
- Europe's top 10 in active clinical trials
- Catalonia stands out among Europe's top 10 in main therapeutic areas