Catalonia, among the top 10 in Europe in clinical trials
<p>Catalonia is among the top 10 countries in Europe in terms of participation in clinical trials and among the top 20 in the world; looking at oncology alone, it is in the European Top 5 and the global Top 10. This November, Barcelona hosted the international Clinical Trials Europe event: a great time to look back over the factors that have made the BioRegion a benchmark in attracting trials from all the major pharmaceutical companies in the world, and also at the future challenges this sector is facing.</p>

The main Catalan research centers have a joint total of over 6,000 active participations in nearly 4,000 clinical trials. In 2017 alone, more than 1,000 trial participations began in Catalonia (one trial may have participations at two or more Catalan centers). If we compare this internationally, Catalonia would be ranked 16th in the world in number of trial participations and 7th in Europe.
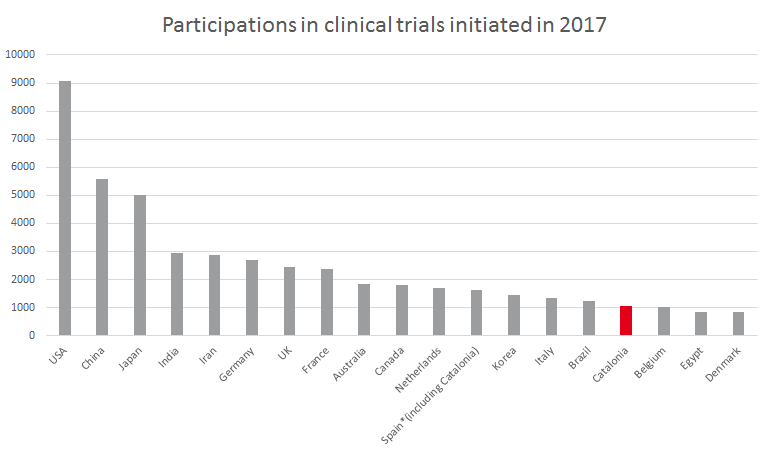
Source: Compiled internally based on data from the UNEIX and WHO
With these figures, Catalonia would be ranked second in the world by number of trials per million inhabitants, behind only Denmark and ahead of countries like the Netherlands and Switzerland.
From November 19 to 21, Barcelona hosted the international Clinical Trials Europe event, which brought together representatives from the global clinical trials arena. “Barcelona is a great center of innovation and scientific research, and an important hub for the rest of Europe,” highlights Louisa Maitland, director of the Clinical Trials Europe conference. “The events highlight Barcelona as a key hub for clinical trials and provides an amazing opportunity for UK companies to present their excellence and expertise and look for collaborations and business opportunities from the thriving biotech and Pharma industry in Barcelona”, said Linda Magee OBE, NHS and Life Sciences Investment Specialist a UK Department for International Trade (DIT).
KOLs and ease of recruitment
Vall d’Hebron, Hospital Clinic, Hospital de Bellvitge, Sant Pau, Parc Taulí and Hospital del Mar are some of the centers in the BioRegion with the most participations in clinical trials. What factors have helped make Catalonia a benchmark destination?
“There are several factors to take into account when deciding which countries will participate in a specific clinical trial, but the most important ones are access to patients and the quality and experience of the centers. Catalonia is well positioned with regard to both of these factors,” explains Tania Nadal, executive director of Global Site Management at TFS Trial Form Support, one of the main international CROs operating in the BioRegion. “In terms of access to patients, compared to Nordic countries, Catalonia offers access to a large population and excellent primary care network. In terms of quality, Catalan research centers, both public and private, are renowned for their experience and facilities,” highlights Nadal. The altruistic tradition among citizens makes recruiting patients much easier than in countries like the United States.
Another main factor is the Key Opinion Leaders (KOLs) who are world renowned in various therapeutic areas: Josep Tabernero in oncology, Jordi Bruix and Josep Maria Llovet in hepatology, Antoni Bayes-Genis in cardiology, Bonaventura Clotet in HIV, Merce Boada in Alzheimer's and Xavier Montalban in multiple sclerosis, to name just a few.
“Another factor is that, in Catalonia, we can do all types of clinical trials, with really good phase I units and access to studies with real-world evidence, which gives us a wide range of opportunities,” notes Tania Nadal, of TFS Trial Form Support. “This, along with the Barcelona brand and good transport connections, creates a pull effect and helps attract more clinical and commercial trials.”
Currently, 11% of clinical trials in Catalonia are phase I, 26% are phase II, 40.3% are phase III and 17.4% are phase IV.
Favorite destination for pharmaceutical corporations
“Barcelona is home to many large global pharmaceutical corporations, not to mention an excellent pool of local pharmaceutical and biotechnology companies, which boosts the region’s global visibility in the industry,” says Tania Nadal, of TFS Trial Form Support.
All of the major global pharmaceutical companies have clinical trials underway in Catalonia, including the top 15 by turnover (Johnson & Johnson, Roche, Pfizer, Novartis, Merck, GSK, Sanofi, AbbVie, Bayer, Eli Lilly, Amgen, Bristol- Myers Squibb, Gilead Sciences, AstraZeneca and Teva).
By number of participations, the most active in the BioRegion are Novartis and Roche. In 2018 alone, Novartis carried out 242 clinical trials with over 3,000 patients in Spain. 164 of these trials were in oncology, and of these 108 were phase I and II. Regarding Roche, Catalan centers bring in more than 2,000 patients, 32% of all participants in the company’s trials in Spain. “The significant weight of Catalonia in Roche’s clinical research is due, among other factors, to the fact that its hospitals are among the top in Spain and the world in many pathologies; the volume of population in Catalonia; that some of these centers are benchmarks for national networks; the presence of national and international KOLs at some of the centers; and units in the early phases that attract a large number of patients,” argues the Medical Department at Roche Pharma.
One of the Catalan pharmaceutical companies that is committed to doing its trials in the local ecosystem is Almirall. “Our clinical studies are global, but being in Catalonia gives us direct access to hospitals with very good researchers who we work closely with, in our case in dermatology,” explains Estrella Garcia, director of Global Clinical Operations at Almirall and one of the speakers at Clinical Trials Europe. “At Almirall, we always try to include Catalan hospitals in our trials, both in the more advanced stages and in proof-of-concept.”
Oncology, the heavyweight
Of the clinical trials conducted in Catalonia, 37.3% are in oncology, followed by those in blood and immune diseases (9.9%), the circulatory system (8.1%), infectious diseases (5.8%) and the digestive system (5.4%), according to data from UNEIX. In fact, based only on participations in oncology trials, Catalonia would be in the European Top 5 and the global Top 10.
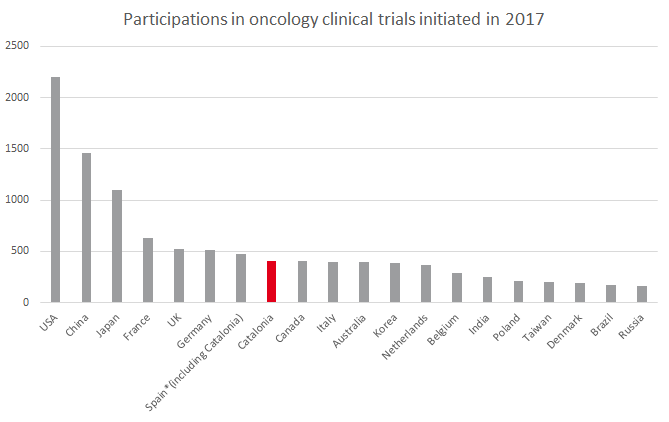
Source: Compiled internally based on data from the UNEIX and WHO
By centers, the Vall d’Hebron Barcelona Hospital Campus is the most active in clinical trials in Catalonia, and one of the most active in Europe. In 2018 alone, this hospital began 306 new trials and recruited more than 1,600 patients, 1,500 in oncology.
Behind these numbers is the work of benchmark researchers like Josep Tabernero, head of Medical Oncology at Vall d’Hebron Hospital in Barcelona, director of the Vall d’Hebron Institute of Oncology (VHIO) and head of the Research Unit for Molecular Therapy of Cancer at Vall d’Hebron Hospital, the first of its kind in Spain to focus on phase I clinical trials in molecular therapy. “The Catalan healthcare system is very favorable for attracting clinical trials: it is easier to recruit patients because the reference systems are well established and the network of hospitals is interconnected, ethics committees are agile, costs are more affordable than in other countries and there are many benchmark researchers pharmaceutical corporations want to work with,” highlights Tabernero.
In fact, he is one of these benchmark names the big companies want to work with, but Tabernero calls attention to the hard work behind it. “It is important for there to be leaders working
to attract innovative trials and that takes a lot of work in terms of international contacts, because there’s a lot of competition among countries,” the oncologist warns. “You have to be dedicated, so if we want to promote more clinical research, it has to be a key goal in our strategy and in results-based management at hospitals, offering incentives for researchers working in this area. The soil is fertile and the system helps, but we need great commitment from clinics and hospital managers by putting clinical research among their priorities,” the expert explains.
The healthcare system’s compensation for this commitment is clear: for example, Vall d’Hebron estimates the hospital saved nearly €26 million last year in oncology medicine by including patients in clinical trials.
On the road to digitalization
According to Kai Langel, Director of Janssen Clinical Innovation, Janssen Research & Development, LLC, the two most prominent trends in terms of the future of clinical trials would be digital endpoints (using digital devices to measure diseases) and decentralized clinical trials: “Today clinical trials are centralized around the clinical trial site, and patients and doctors need to be there. Thanks to telemedicine and sensors the model can be flexible and improve access to clinical trials for patients not living in big cities”. The Innovative Medicines Initiative has launched a large project called Trials@Home to develop an operational framework for this model in Europe.
Kai Langel is currently based in Tarragona and, according to his experience, Spain in general and Catalonia in particular are leading interesting initiatives regarding such next-generation approaches. “Spain is currently quite progressive in Europe in this field and Janssen has strategic collaborations here. You can find many industry stakeholders working together in Catalonia, and there is a very innovative spirit. Moreover, Catalonia is a hot bed for mobile technology innovation, with Mobile World Congress generating an ecosystem of mobile technology startups that such advancements, so it is definitely a good place to be if you work in cutting edge innovation”.
Langel gives Almirall’s experience with digitalization as an example. “We’re working to digitalize the whole company, but specifically in the clinical trials area we’re moving towards hybrid trials that include remote appointments with patients using an app on their phone, where they can even sign their informed consent sheets,” explains Estrella Garcia, director of Global Clinical Operations at Almirall. “Catalan hospitals are very prepared to participate in this type of study and, in fact, Catalonia is already taking part in two hybrid trials we have underway, with 30% of trial appointments taking place remotely via messages or videoconferences.”
Future challenges
In Catalonia, clinical studies have proven to be beneficial not only to residents, giving them access to pharmaceutical innovations, but also to the system’s sustainability.
However, there are still many challenges to be tackled. For years now, the pharmaceutical industry, clinics, CROs and regulatory agencies have warned that the current clinical trial model isn’t sustainable and should be re-conceived to cut costs and red tape, optimize design (more flexible, more inclusive, more cooperative, more ambitious) and adapt to patients’ needs.
In this framework, how can Catalonia continue promoting clinical research? “First, we have to change our culture of competition for one of cooperation; second, we need to make the most of our strengths (innovation assets in the system, interconnected network of healthcare devices, participation culture, professional leadership); and third, we have to promote all clinical research, not only in pharmacology but also in cell therapy, regenerative or gene therapy, healthcare products, medical devices, telemedicine, wearables, apps,” argues Cristina Nadal, the new director of Healthcare Programs at Biocat.


